Argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas.[9] Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust.
This article is about the chemical element. For other uses, see Argon (disambiguation).Argon
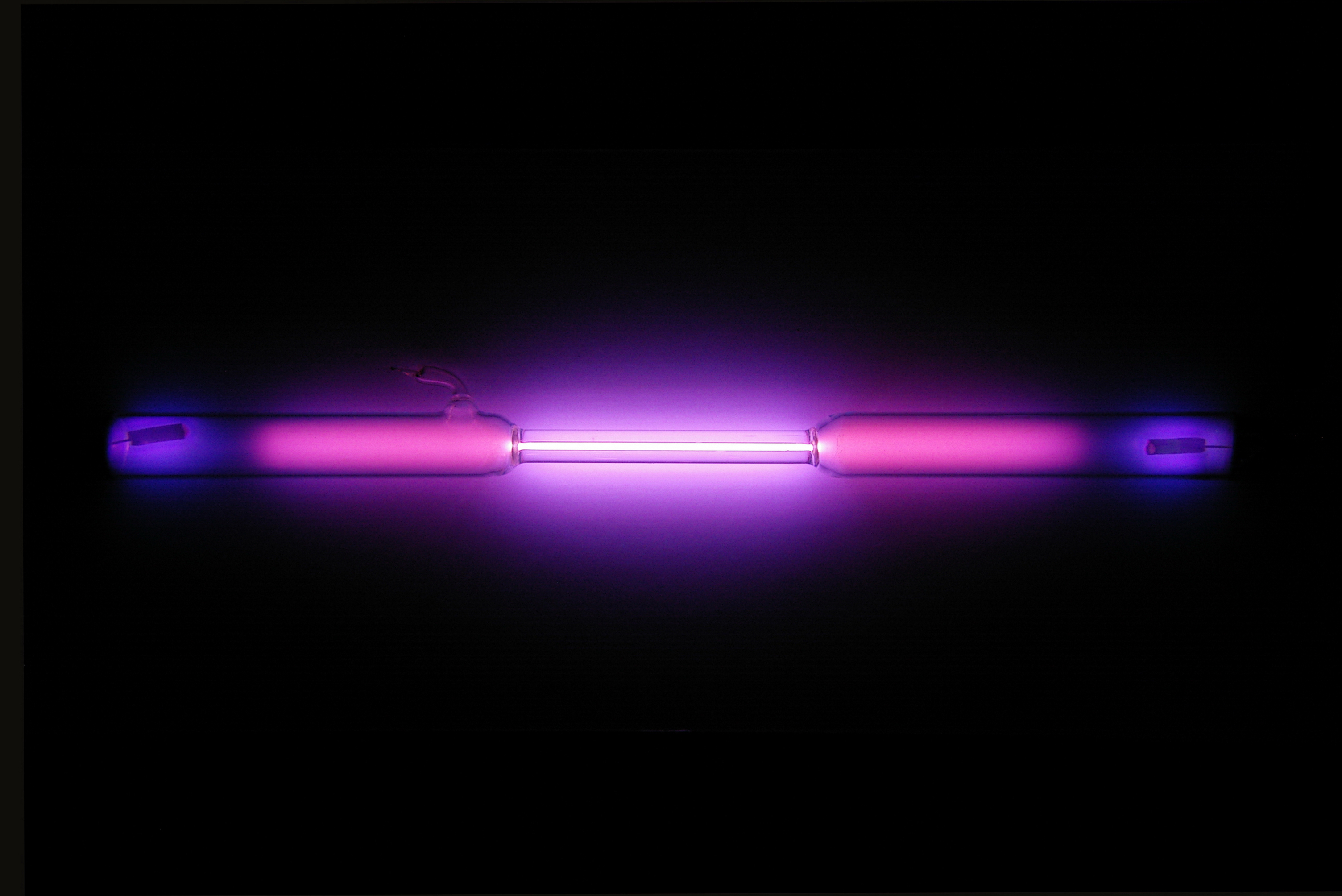
colorless gas exhibiting a lilac/violet glow when placed in an electric field
$_$_$DEEZ_NUTS#1__answer--2DEEZ_NUTS$_$_$
18
[Ne] 3s2 3p6
2, 8, 8
83.81 K (−189.34 °C, −308.81 °F)
87.302 K (−185.848 °C, −302.526 °F)
1.784 g/L
1.3954 g/cm3
83.8058 K, 68.89 kPa[3]
150.687 K, 4.863 MPa[3]
1.18 kJ/mol
6.53 kJ/mol
20.85[4] J/(mol·K)
0
Pauling scale: no data
- 1st: 1520.6 kJ/mol
- 2nd: 2665.8 kJ/mol
- 3rd: 3931 kJ/mol
- (more)
106±10 pm
188 pm
face-centered cubic (fcc) (cF4)
17.72×10−3 W/(m⋅K)
−19.6×10−6 cm3/mol[7]
323 m/s (gas, at 27 °C)
7440-37-1
Lord Rayleigh and William Ramsay (1894)
Nearly all argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas.
The name "argon" is derived from the Greek word ἀργόν, neuter singular form of ἀργός meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The complete octet (eight electrons) in the outer atomic shell makes argon stable and resistant to bonding with other elements. Its triple point temperature of 83.8058 K is a defining fixed point in the International Temperature Scale of 1990.
Argon is extracted industrially by the fractional distillation of liquid air. It is mostly used as an inert shielding gas in welding and other high-temperature industrial processes where ordinarily unreactive substances become reactive; for example, an argon atmosphere is used in graphite electric furnaces to prevent the graphite from burning. It is also used in incandescent, fluorescent lighting, and other gas-discharge tubes. It makes a distinctive blue-green gas laser. It is also used in fluorescent glow starters.
Occurrence
Argon constitutes 0.934% by volume and 1.288% by mass of Earth's atmosphere.[24] Air is the primary industrial source of purified argon products. Argon is isolated from air by fractionation, most commonly by cryogenic fractional distillation, a process that also produces purified nitrogen, oxygen, neon, krypton and xenon.[25] Earth's crust and seawater contain 1.2 ppm and 0.45 ppm of argon, respectively.[26]
Production
Argon is extracted industrially by the fractional distillation of liquid air in a cryogenic air separation unit; a process that separates liquid nitrogen, which boils at 77.3 K, from argon, which boils at 87.3 K, and liquid oxygen, which boils at 90.2 K. About 700,000 tonnes of argon are produced worldwide every year.[26][38]
Safety
Although argon is non-toxic, it is 38% more dense than air and therefore considered a dangerous asphyxiant in closed areas. It is difficult to detect because it is colorless, odorless, and tasteless. A 1994 incident, in which a man was asphyxiated after entering an argon-filled section of oil pipe under construction in Alaska, highlights the dangers of argon tank leakage in confined spaces and emphasizes the need for proper use, storage and handling.[55]
