Ocean acidification
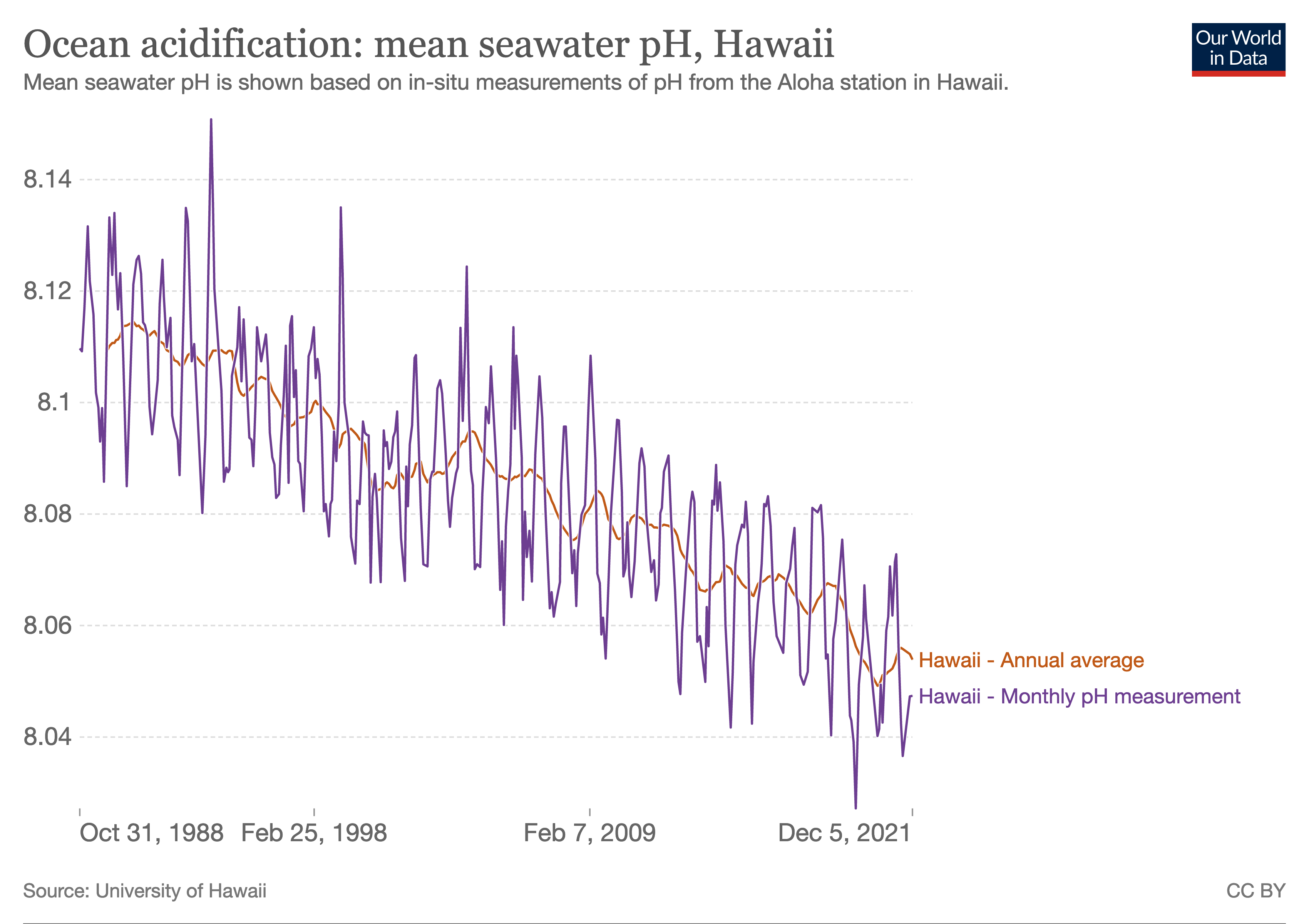
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05.[2] Carbon dioxide emissions from human activities are the primary cause of ocean acidification, with atmospheric carbon dioxide (CO2) levels exceeding 410 ppm (in 2020). CO2 from the atmosphere is absorbed by the oceans. This chemical reaction produces carbonic acid (H2CO3) which dissociates into a bicarbonate ion (HCO−3) and a hydrogen ion (H+). The presence of free hydrogen ions (H+) lowers the pH of the ocean, increasing acidity (this does not mean that seawater is acidic yet; it is still alkaline, with a pH higher than 8). Marine calcifying organisms, such as mollusks and corals, are especially vulnerable because they rely on calcium carbonate to build shells and skeletons.[3]
A change in pH by 0.1 represents a 26% increase in hydrogen ion concentration in the world's oceans (the pH scale is logarithmic, so a change of one in pH units is equivalent to a tenfold change in hydrogen ion concentration). Sea-surface pH and carbonate saturation states vary depending on ocean depth and location. Colder and higher latitude waters are capable of absorbing more CO2. This can cause acidity to rise, lowering the pH and carbonate saturation levels in these areas. Other factors that influence the atmosphere-ocean CO2 exchange, and thus local ocean acidification, include: ocean currents and upwelling zones, proximity to large continental rivers, sea ice coverage, and atmospheric exchange with nitrogen and sulfur from fossil fuel burning and agriculture.[4][5][6]
Decreased ocean pH has a range of potentially harmful effects for marine organisms. These include reduced calcification, depressed metabolic rates, lowered immune responses, and reduced energy for basic functions such as reproduction.[7] The effects of ocean acidification are therefore impacting marine ecosystems that provide food, livelihoods, and other ecosystem services for a large portion of humanity. Some 1 billion people are wholly or partially dependent on the fishing, tourism, and coastal management services provided by coral reefs. Ongoing acidification of the oceans may therefore threaten food chains linked with the oceans.[8][9]
The United Nations Sustainable Development Goal 14 ("Life below Water") has a target to "minimize and address the impacts of ocean acidification".[10] Reducing carbon dioxide emissions (i.e., climate change mitigation measures) is the only solution that addresses the root cause of ocean acidification. Mitigation measures which achieve carbon dioxide removal from the atmosphere would help to reverse ocean acidification. The more specific ocean-based mitigation methods (e.g. ocean alkalinity enhancement, enhanced weathering) could also reduce ocean acidification. These strategies are being researched, but generally have a low technology readiness level and many risks.[11][12][13]
Ocean acidification has occurred previously in Earth's history.[14] The resulting ecological collapse in the oceans had long-lasting effects on the global carbon cycle and climate.
History[edit]
Research into the phenomenon of ocean acidification, as well as awareness raising about the problem, has been going on for several decades. The fundamental research really began with the creation of the pH scale by Danish chemist Søren Peder Lauritz Sørensen in 1909.[173] By around the 1950s the massive role of the ocean in absorbing fossil fuel CO2 was known to specialists, but not appreciated by the greater scientific community.[174] Throughout much of the 20th century, the dominant focus has been the beneficial process of oceanic CO2 uptake, which has enormously ameliorated climate change. The concept of "too much of a good thing" has been late in developing and was triggered only by some key events, and the oceanic sink for heat and CO2 is still critical as the primary buffer against climate change.[174]
In the early 1970s questions over the long-term impact of the accumulation of fossil fuel CO2 in the sea were already arising around the world and causing strong debate. Researchers commented on the accumulation of fossil CO2 in the atmosphere and sea and drew attention to the possible impacts on marine life. By the mid-1990s, the likely impact of CO2 levels rising so high with the inevitable changes in pH and carbonate ion became a concern of scientists studying the fate of coral reefs.[174]
By the end of the 20th century the trade-offs between the beneficial role of the ocean in absorbing some 90 % of all heat created, and the accumulation of some 50 % of all fossil fuel CO2 emitted, and the impacts on marine life were becoming more clear. By 2003, the time of planning for the "First Symposium on the Ocean in a High-CO2 World" meeting to be held in Paris in 2004, many new research results on ocean acidification were published.[174]
In 2009, members of the InterAcademy Panel called on world leaders to "Recognize that reducing the build up of CO2 in the atmosphere is the only practicable solution to mitigating ocean acidification".[175] The statement also stressed the importance to "Reinvigorate action to reduce stressors, such as overfishing and pollution, on marine ecosystems to increase resilience to ocean acidification".[176]
For example, research in 2010 found that in the 15-year period 1995–2010 alone, acidity had increased 6 percent in the upper 100 meters of the Pacific Ocean from Hawaii to Alaska.[48]
According to a statement in July 2012 by Jane Lubchenco, head of the U.S. National Oceanic and Atmospheric Administration "surface waters are changing much more rapidly than initial calculations have suggested. It's yet another reason to be very seriously concerned about the amount of carbon dioxide that is in the atmosphere now and the additional amount we continue to put out."[177]
A 2013 study found acidity was increasing at a rate 10 times faster than in any of the evolutionary crises in Earth's history.[178]
The "Third Symposium on the Ocean in a High-CO2 World" took place in Monterey, California, in 2012. The summary for policy makers from the conference stated that "Ocean acidification research is growing rapidly".[94]
In a synthesis report published in Science in 2015, 22 leading marine scientists stated that CO2 from burning fossil fuels is changing the oceans' chemistry more rapidly than at any time since the Great Dying (Earth's most severe known extinction event).[157] Their report emphasized that the 2 °C maximum temperature increase agreed upon by governments reflects too small a cut in emissions to prevent "dramatic impacts" on the world's oceans.[157]