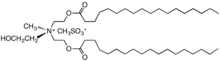
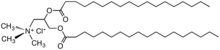
Quaternary ammonium cation
In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure [NR4]+, where R is an alkyl group, an aryl group[1] or organyl group. Unlike the ammonium ion (NH+4) and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.
Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces than bleach-based disinfectants, and are generally fabric-safe.[2]
Quantification[edit]
The quantification of quaternary ammonium compounds can be challenging. Some methods include precipitation of solid salts with tetraphenylborate. Another method, an Epton titration, involves partitioning between water-chloroform in the presence of an anionic dye. Individual cations are detectable by ESI-MS and NMR spectroscopy.[4]