Sulfur hexafluoride
Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non-flammable, and non-toxic gas. SF
6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule.
Typical for a nonpolar gas, SF
6 is poorly soluble in water but quite soluble in nonpolar organic solvents. It has a density of 6.12 g/L at sea level conditions, considerably higher than the density of air (1.225 g/L). It is generally transported as a liquefied compressed gas.
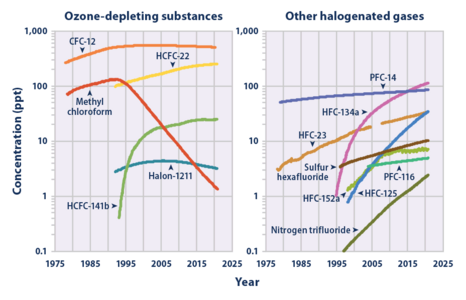
SF
6 has 23,500 times greater global warming potential (GWP) than CO2 as a greenhouse gas (over a 100-year time-frame) but exists in relatively minor concentrations in the atmosphere. Its concentration in Earth's troposphere reached 11.50 parts per trillion (ppt) in October 2023, rising at 0.37 ppt/year.[8] The increase since 1980 is driven in large part by the expanding electric power sector, including fugitive emissions from banks of SF
6 gas contained in its medium- and high-voltage switchgear. Uses in magnesium, aluminium, and electronics manufacturing also hastened atmospheric growth.[9] The 1997 Kyoto Protocol, which came into force in 2005, is supposed to limit emissions of this gas. In a somewhat nebulous way it has been included as part of the carbon emission trading scheme. In some countries this has led to the defunction of entire industries.[10]
According to the Intergovernmental Panel on Climate Change, SF
6 is the most potent greenhouse gas. Its global warming potential of 23,900 times that of CO
2 when compared over a 100-year period.[42] Sulfur hexafluoride is inert in the troposphere and stratosphere and is extremely long-lived, with an estimated atmospheric lifetime of 800–3,200 years.[43]
Measurements of SF6 show that its global average mixing ratio has increased from a steady base of about 54 parts per quadrillion[11] prior to industrialization, to over 11.5 parts per trillion (ppt) as of October 2023, and is increasing by about 0.4 ppt (3.5%) per year.[8][44] Average global SF6 concentrations increased by about 7% per year during the 1980s and 1990s, mostly as the result of its use in magnesium production, and by electrical utilities and electronics manufacturers. Given the small amounts of SF6 released compared to carbon dioxide, its overall individual contribution to global warming is estimated to be less than 0.2%,[45] however the collective contribution of it and similar man-made halogenated gases has reached about 10% as of 2020.[46] Alternatives are being tested.[47][48]
In Europe, SF
6 falls under the F-Gas directive which ban or control its use for several applications.[49] Since 1 January 2006, SF
6 is banned as a tracer gas and in all applications except high-voltage switchgear.[50] It was reported in 2013 that a three-year effort by the United States Department of Energy to identify and fix leaks at its laboratories in the United States such as the Princeton Plasma Physics Laboratory, where the gas is used as a high voltage insulator, had been productive, cutting annual leaks by 1,030 kilograms (2,280 pounds). This was done by comparing purchases with inventory, assuming the difference was leaked, then locating and fixing the leaks.[51]

![Abundance and growth rate of SF 6 in Earth's troposphere (1978-2018).[9]](http://upload.wikimedia.org/wikipedia/commons/thumb/7/7b/AGAGE_sulfur_hexafluroride_growth.png/404px-AGAGE_sulfur_hexafluroride_growth.png)