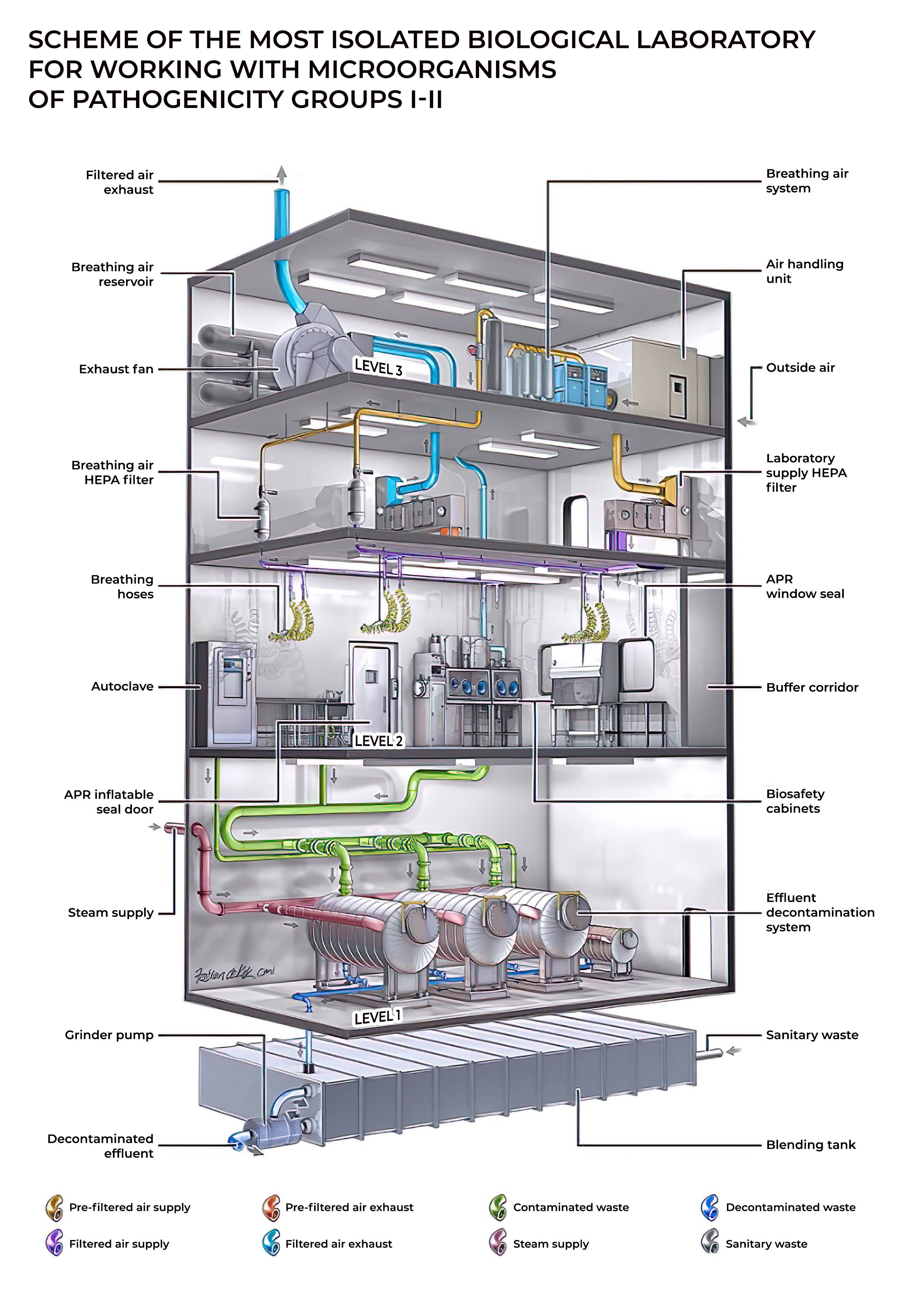
Biosafety level
A biosafety level (BSL), or pathogen/protection level, is a set of biocontainment precautions required to isolate dangerous biological agents in an enclosed laboratory facility. The levels of containment range from the lowest biosafety level 1 (BSL-1) to the highest at level 4 (BSL-4). In the United States, the Centers for Disease Control and Prevention (CDC) have specified these levels in a publication referred to as BMBL.[2] In the European Union, the same biosafety levels are defined in a directive.[3] In Canada the four levels are known as Containment Levels.[4] Facilities with these designations are also sometimes given as P1 through P4 (for pathogen or protection level), as in the term P3 laboratory.[5]
At the lowest level of biosafety, precautions may consist of regular hand-washing and minimal protective equipment. At higher biosafety levels, precautions may include airflow systems, multiple containment rooms, sealed containers, positive pressure personnel suits, established protocols for all procedures, extensive personnel training, and high levels of security to control access to the facility. Health Canada reports that world-wide until 1999 there were recorded over 5,000 cases of accidental laboratory infections and 190 deaths.[6]
History[edit]
The first prototype Class III (maximum containment) biosafety cabinet was fashioned in 1943 by Hubert Kaempf Jr., then a U.S. Army soldier, under the direction of Arnold G. Wedum, Director (1944–1969) of Industrial Health and Safety at the United States Army Biological Warfare Laboratories, Camp Detrick, Maryland. Kaempf was tired of his MP duties at Detrick and was able to transfer to the sheet metal department working with the contractor, the H.K. Ferguson Co.[7]
On 18 April 1955, fourteen representatives met at Camp Detrick in Frederick, Maryland. The meeting was to share knowledge and experiences regarding biosafety, chemical, radiological, and industrial safety issues that were common to the operations at the three principal biological warfare (BW) laboratories of the U.S. Army.[8] Because of the potential implication of the work conducted at biological warfare laboratories, the conferences were restricted to top level security clearances. Beginning in 1957, these conferences were planned to include non-classified sessions as well as classified sessions to enable broader sharing of biological safety information. It was not until 1964, however, that conferences were held in a government installation not associated with a biological warfare program.[9]
Over the next ten years, the biological safety conferences grew to include representatives from all federal agencies that sponsored or conducted research with pathogenic microorganisms. By 1966, it began to include representatives from universities, private laboratories, hospitals, and industrial complexes. Throughout the 1970s, participation in the conferences continued to expand and by 1983 discussions began regarding the creation of a formal organization.[9] The American Biological Safety Association (ABSA) was officially established in 1984 and a constitution and bylaws were drafted the same year. As of 2008, ABSA includes some 1,600 members in its professional association.[9]
In 1977, Jim Peacock of the Australian Academy of Science asked Bill Snowdon, then chief of the CSIRO's Australian Animal Health Laboratory (AAHL) if he could have the newly released United States' National Institutes of Health and the British equivalent requirements for the development of infrastructure for bio-containment reviewed by AAHL personnel with a view to recommending the adoption of one of them by Australian authorities. The review was carried out by CSIRO AAHL Project Manager Bill Curnow and CSIRO Engineer Arthur Jenkins. They drafted outcomes for each of the levels of security. AAHL was notionally classified as "substantially beyond P4". These were adopted by the Australian Academy of Science and became the basis for Australian legislation. It opened in 1985 costing AU$185 million, built on Corio Oval.[10] The Australian Animal Health Laboratory is a Class 4/ P4 Laboratory.[11][12]
In 2003, the Chinese Academy of Sciences approved the construction of mainland China's first BSL-4 laboratory at the Wuhan Institute of Virology (WIV). In 2014, the WIV's National Bio-safety Laboratory was built at a cost of 300 million yuan (US$44 million), in collaboration and with assistance from the French government's CIRI lab.[13][14][15]
In 2007 a scientific review paper stated that the Canadian Science Centre for Human and Animal Health, which was designed in the early 1990s, "has become the prototype for modern BSL4 laboratories".[16]
Starting with the 2020 COVID-19 pandemic near the facilities of the WIV, work in biocontainment facilities has been politicized, especially in the US Senate for example as the result of Rand Paul's work.[17] Russia asked questions on 25 October 2022 in the United Nations over the presence in Ukraine of biolabs.[18] In April 2023, Sudan's descent into civil war caused worries at the World Health Organization over its National Public Laboratory as contending factions battled over its area and NPL staff were kicked out in favor of installing a military base at its premises.[19] At the time, the facility contained organisms rated at BSL-2.[20]
Levels[edit]
Biosafety level 1[edit]
Biosafety level 1 (BSL-1) is suitable for work with well-characterized agents which do not cause disease in healthy humans. In general, these agents should pose minimal potential hazard to laboratory personnel and the environment.[21] At this level, precautions are limited relative to other levels. Laboratory personnel must wash their hands upon entering and exiting the lab. Research with these agents may be performed on standard open laboratory benches without the use of special containment equipment. However, eating and drinking are generally prohibited in laboratory areas.[21] Potentially infectious material must be decontaminated before disposal, either by adding a chemical such as bleach or isopropanol or by packaging for decontamination elsewhere.[21] Personal protective equipment is only required for circumstances where personnel might be exposed to hazardous material.[21] BSL-1 laboratories must have a door which can be closed to limit access to the lab. However, it is not necessary for BSL-1 labs to be isolated from the general building.[22]
This level of biosafety is appropriate for work with several kinds of microorganisms including non-pathogenic strains of Escherichia coli and Staphylococcus, Bacillus subtilis, Saccharomyces cerevisiae and other organisms not suspected to contribute to human disease.[23] Due to the relative ease and safety of maintaining a BSL-1 laboratory, these are the types of laboratories generally used as teaching spaces for high schools and colleges.[22]
Biosafety level 2[edit]
At this level, all precautions used at Biosafety level 1 are followed, and some additional precautions are taken. BSL-2 differs from BSL-1 in that:
Safety concerns[edit]
A North Carolina Mosquito & Vector Control Association (NCMVCA) study highlighted safety concerns. In the United States, laboratories can be funded by federal, state, private, non-profit, or academically. The last accounts for 72% of the funding.[100]
High-containment labs that are registered with the Centers for Disease Control and Prevention (CDC) and the U.S. Department of Agriculture's (USDA) Select Agent Program must adhere to Department of Defense standards.[101] Since BSL3 and 4 laboratories in the United States are regulated by either the CDC or USDA or another federal agency (depending on the pathogens they handle), no single federal agency is responsible for regulating or tracking the number of these labs.[102] U.S. high-containment laboratories that handle pathogens which are declared as "select agents" must be inspected periodically by the CDC or USDA, adhere to certain standards, and maintain ongoing education on biosecurity and biosafety policies as mandated by law.[103][104]