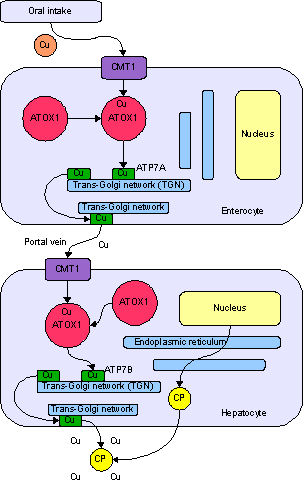
Copper in biology
Copper is an essential trace element that is vital to the health of all living things (plants, animals and microorganisms). In humans, copper is essential to the proper functioning of organs and metabolic processes. Also, in humans, copper helps maintain the nervous system, immune system, brain development, and activates genes, as well as assisting in the production of connective tissues, blood vessels, and energy.[1] The human body has complex homeostatic mechanisms which attempt to ensure a constant supply of available copper, while eliminating excess copper whenever this occurs. However, like all essential elements and nutrients, too much or too little nutritional ingestion of copper can result in a corresponding condition of copper excess or deficiency in the body, each of which has its own unique set of adverse health effects.
Daily dietary standards for copper have been set by various health agencies around the world. Standards adopted by some nations recommend different copper intake levels for adults, pregnant women, infants, and children, corresponding to the varying need for copper during different stages of life.
Copper proteins have diverse roles in biological electron transport and oxygen transportation, processes that exploit the easy interconversion of Cu(I) and Cu(II).[2] Copper is essential in the aerobic respiration of all eukaryotes. In mitochondria, it is found in cytochrome c oxidase, which is the last protein in oxidative phosphorylation. Cytochrome c oxidase is the protein that binds the O2 between a copper and an iron; the protein transfers 4 electrons to the O2 molecule to reduce it to two molecules of water. Copper is also found in many superoxide dismutases, proteins that catalyze the decomposition of superoxides by converting it (by disproportionation) to oxygen or hydrogen peroxide:
The protein hemocyanin is the oxygen carrier in most mollusks and some arthropods such as the horseshoe crab (Limulus polyphemus).[3] Because hemocyanin is blue, these organisms have blue blood rather than the red blood of iron-based hemoglobin. Structurally related to hemocyanin are the laccases and tyrosinases. Instead of reversibly binding oxygen, these proteins hydroxylate substrates, illustrated by their role in the formation of lacquers.[4] The biological role for copper commenced with the appearance of oxygen in Earth's atmosphere.[5] Several copper proteins, such as the "blue copper proteins", do not interact directly with substrates; hence they are not enzymes. These proteins relay electrons by the process called electron transfer.[4]
A unique tetranuclear copper center has been found in nitrous-oxide reductase.[6]
Chemical compounds which were developed for treatment of Wilson's disease have been investigated for use in cancer therapy.[7]
Optimal copper levels[edit]
Copper deficiency and toxicity can be either of genetic or non-genetic origin. The study of copper's genetic diseases, which are the focus of intense international research activity, has shed insight into how human bodies use copper, and why it is important as an essential micronutrient. The studies have also resulted in successful treatments for genetic copper excess conditions, empowering patients whose lives were once jeopardized.
Researchers specializing in the fields of microbiology, toxicology, nutrition, and health risk assessments are working together to define the precise copper levels that are required for essentiality, while avoiding deficient or excess copper intakes. Results from these studies are expected to be used to fine-tune governmental dietary recommendation programs which are designed to help protect public health.
Cancer[edit]
The role of copper in angiogenesis associated with different types of cancers has been investigated.[125] A copper chelator, tetrathiomolybdate, which depletes copper stores in the body, is under investigation as an anti-angiogenic agent in pilot[126] and clinical trials.[127] The drug may inhibit tumor angiogenesis in hepatocellular carcinoma, pleural mesothelioma, colorectal cancer, head and neck squamous cell carcinoma, breast cancer, and kidney cancer.[128] The copper complex of a synthetic salicylaldehyde pyrazole hydrazone (SPH) derivative induced human umbilical endothelial cell (HUVEC) apoptosis and showed anti-angiogenesis effect in vitro.[129]
The trace element copper had been found promoting tumor growth.[130][131] Several evidence from animal models indicates that tumors concentrate high levels of copper. Meanwhile, extra copper has been found in some human cancers.[132][133] Recently, therapeutic strategies targeting copper in the tumor have been proposed. Upon administration with a specific copper chelator, copper complexes would be formed at a relatively high level in tumors. Copper complexes are often toxic to cells, therefore tumor cells were killed, while normal cells in the whole body remained alive for the lower level of copper.[134] Researchers have also recently found that cuproptosis, a copper-induced mechanism of mitochondrial-related cell death, has been implicated as a breakthrough in the treatment of cancer and has become a new treatment strategy. [135]
Some copper chelators get more effective or novel bioactivity after forming copper-chelator complexes. It was found that Cu2+ was critically needed for PDTC induced apoptosis in HL-60 cells.[136] The copper complex of salicylaldehyde benzoylhydrazone (SBH) derivatives showed increased efficacy of growth inhibition in several cancer cell lines, when compared with the metal-free SBHs.[137][138][139]
SBHs can react with many kinds of transition metal cations and thereby forming a number of complexes.[139][140][141] Copper-SBH complexes were more cytotoxic than complexes of other transitional metals (Cu > Ni > Zn = Mn > Fe = Cr > Co) in MOLT-4 cells, an established human T-cell leukemia cell line. SBHs, especially their copper complexes appeared to be potent inhibitors of DNA synthesis and cell growth in several human cancer cell lines, and rodent cancer cell lines.[137][138]
Salicylaldehyde pyrazole hydrazone (SPH) derivatives were found to inhibit the growth of A549 lung carcinoma cells.[142] SPH has identical ligands for Cu2+ as SBH. The Cu-SPH complex was found to induce apoptosis in A549, H322 and H1299 lung cancer cells.[143]