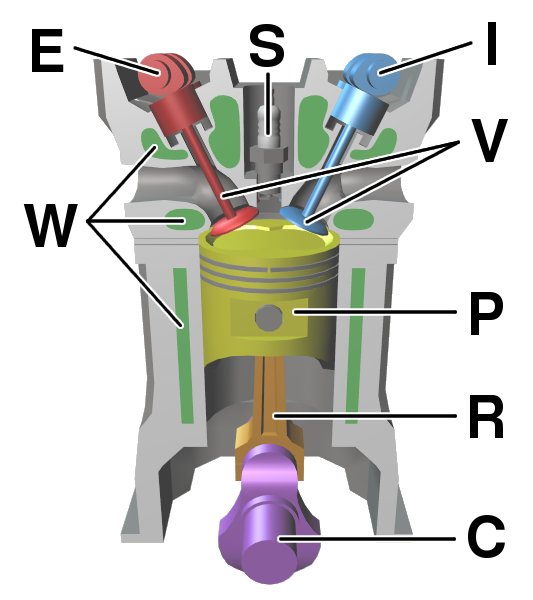
Internal combustion engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons (piston engine), turbine blades (gas turbine), a rotor (Wankel engine), or a nozzle (jet engine). This force moves the component over a distance, transforming chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to.
"ICEV" redirects here. For the form of water ice, see Ice V. For the high speed train, see ICE V.
The first commercially successful internal combustion engine was created by Étienne Lenoir around 1860,[1] and the first modern internal combustion engine, known as the Otto engine, was created in 1876 by Nicolaus Otto. The term internal combustion engine usually refers to an engine in which combustion is intermittent, such as the more familiar two-stroke and four-stroke piston engines, along with variants, such as the six-stroke piston engine and the Wankel rotary engine. A second class of internal combustion engines use continuous combustion: gas turbines, jet engines and most rocket engines, each of which are internal combustion engines on the same principle as previously described.[1][2] (Firearms are also a form of internal combustion engine,[2] though of a type so specialized that they are commonly treated as a separate category, along with weaponry such as mortars and anti-aircraft cannons.) In contrast, in external combustion engines, such as steam or Stirling engines, energy is delivered to a working fluid not consisting of, mixed with, or contaminated by combustion products. Working fluids for external combustion engines include air, hot water, pressurized water or even boiler-heated liquid sodium.
While there are many stationary applications, most ICEs are used in mobile applications and are the primary power supply for vehicles such as cars, aircraft and boats. ICEs are typically powered by hydrocarbon-based fuels like natural gas, gasoline, diesel fuel, or ethanol. Renewable fuels like biodiesel are used in compression ignition (CI) engines and bioethanol or ETBE (ethyl tert-butyl ether) produced from bioethanol in spark ignition (SI) engines. As early as 1900 the inventor of the diesel engine, Rudolf Diesel, was using peanut oil to run his engines.[3] Renewable fuels are commonly blended with fossil fuels. Hydrogen, which is rarely used, can be obtained from either fossil fuels or renewable energy.
Etymology[edit]
At one time, the word engine (via Old French, from Latin ingenium, "ability") meant any piece of machinery—a sense that persists in expressions such as siege engine. A "motor" (from Latin motor, "mover") is any machine that produces mechanical power. Traditionally, electric motors are not referred to as "engines"; however, combustion engines are often referred to as "motors". (An electric engine refers to a locomotive operated by electricity.)
In boating, an internal combustion engine that is installed in the hull is referred to as an engine, but the engines that sit on the transom are referred to as motors.[15]
Internal combustion engines must have their cycles started. In reciprocating engines this is accomplished by turning the crankshaft (Wankel Rotor Shaft) which induces the cycles of intake, compression, combustion, and exhaust. The first engines were started with a turn of their flywheels, while the first vehicle (the Daimler Reitwagen) was started with a hand crank. All ICE engined automobiles were started with hand cranks until Charles Kettering developed the electric starter for automobiles.[47] This method is now the most widely used, even among non-automobiles.
As diesel engines have become larger and their mechanisms heavier, air starters have come into use.[48] This is due to the lack of torque in electric starters. Air starters work by pumping compressed air into the cylinders of an engine to start it turning.
Two-wheeled vehicles may have their engines started in one of four ways:
There are also starters where a spring is compressed by a crank motion and then used to start an engine.
Some small engines use a pull-rope mechanism called "recoil starting", as the rope rewinds itself after it has been pulled out to start the engine. This method is commonly used in pushed lawn mowers and other settings where only a small amount of torque is needed to turn an engine over.
Turbine engines are frequently started by an electric motor or by compressed air.
Carbon dioxide formation[edit]
A good way to estimate the mass of carbon dioxide that is released when one litre of diesel fuel (or gasoline) is combusted can be found as follows:[52]
As a good approximation the chemical formula of diesel is C
nH
2n. In reality diesel is a mixture of different molecules. As carbon has a molar mass of 12 g/mol and hydrogen (atomic) has a molar mass of about 1 g/mol, the fraction by weight of carbon in diesel is roughly 12⁄14.
The reaction of diesel combustion is given by:
2C
nH
2n + 3nO
2 ⇌ 2nCO
2 + 2nH
2O
Carbon dioxide has a molar mass of 44 g/mol as it consists of 2 atoms of oxygen (16 g/mol) and 1 atom of carbon (12 g/mol). So 12 g of carbon yields 44 g of carbon dioxide.
Diesel has a density of 0.838 kg per litre.
Putting everything together the mass of carbon dioxide that is produced by burning 1 litre of diesel can be calculated as:
The figure obtained with this estimation is close to the values found in the literature.
For gasoline, with a density of 0.75 kg/L and a ratio of carbon to hydrogen atoms of about 6 to 14, the estimated value of carbon dioxide emission from burning 1 litre of gasoline is:

