Lennox–Gastaut syndrome
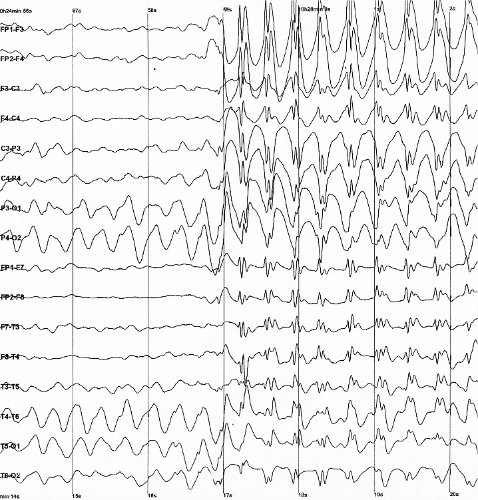
Lennox–Gastaut syndrome (LGS) is a complex, rare, and severe childhood-onset epilepsy syndrome. It is characterized by multiple and concurrent seizure types including tonic seizure, cognitive dysfunction, and slow spike waves on electroencephalogram (EEG), which are very abnormal.[1] Typically, it presents in children aged 3–5 years and most of the time persists into adulthood with slight changes in the electroclinical phenotype.[2][3] It has been associated with perinatal injuries, congenital infections, brain malformations, brain tumors, genetic disorders such as tuberous sclerosis and numerous gene mutations. Sometimes LGS is observed after infantile epileptic spasm syndrome (formerly called West syndrome). The prognosis for LGS is marked by a 5% mortality in childhood and persistent seizures into adulthood (at least 90% of adults with LGS still have seizures).[4]
Lennox–Gastaut syndrome
LGS was named for neurologists William G. Lennox (Boston, US) and Henri Gastaut (Marseille, France),[5] who independently described the condition. The international LGS Awareness Day is on November 1.[6]
Research[edit]
Vigabatrin was found by Feucht et al. to be an effective add-on in patients whose seizures were not satisfactorily controlled by valproate. Out of 20 children, only 1 experienced a serious side effect (dyskinesia).[39]
Zonisamide showed promise in an overview of controlled and uncontrolled trials conducted in Japan.[40] However, in a physician survey conducted December 2004, only 28% of Lennox–Gastaut and West syndrome patients improved on zonisamide.[41]
Soticlestat is an investigational anticonvulsant that was well tolerated and reduced seizure frequency in a phase 2 clinical study for the treatment of Lennox-Gastaut syndrome[42][43][44] and began phase 3 trials in 2022.[44]
Lennox-Gastaut Syndrome Foundation[edit]
The Lennox-Gastaut Syndrome (LGS) Foundation, based in San Diego, California, is dedicated to improving the lives of those impacted by LGS through advancing research, awareness, education, and family support. The organization's slogan is: "The challenges are tough. So are we".[45]