Polycyclic aromatic hydrocarbon
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the incomplete combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.
Polycyclic aromatic hydrocarbons are discussed as possible starting materials for abiotic syntheses of materials required by the earliest forms of life.[1][2]
The aromaticity varies for PAHs. According to Clar's rule,[17] the resonance structure of a PAH that has the largest number of disjoint aromatic pi sextets—i.e. benzene-like moieties—is the most important for the characterization of the properties of that PAH.[18]
For example, phenanthrene has two Clar structures: one with just one aromatic sextet (the middle ring), and the other with two (the first and third rings). The latter case is therefore the more characteristic electronic nature of the two. Therefore, in this molecule the outer rings have greater aromatic character whereas the central ring is less aromatic and therefore more reactive. In contrast, in anthracene the resonance structures have one sextet each, which can be at any of the three rings, and the aromaticity spreads out more evenly across the whole molecule. This difference in number of sextets is reflected in the differing ultraviolet–visible spectra of these two isomers, as higher Clar pi-sextets are associated with larger HOMO-LUMO gaps;[19] the highest-wavelength absorbance of phenanthrene is at 293 nm, while anthracene is at 374 nm.[20] Three Clar structures with two sextets each are present in the four-ring chrysene structure: one having sextets in the first and third rings, one in the second and fourth rings, and one in the first and fourth rings. Superposition of these structures reveals that the aromaticity in the outer rings is greater (each has a sextet in two of the three Clar structures) compared to the inner rings (each has a sextet in only one of the three).
Properties[edit]
Physicochemical[edit]
PAHs are nonpolar and lipophilic. Larger PAHs are generally insoluble in water, although some smaller PAHs are soluble.[21][22] The larger members are also poorly soluble in organic solvents and in lipids. The larger members, e.g. perylene, are strongly colored.[16]
Redox[edit]
Polycyclic aromatic compounds characteristically yield radicals and anions upon treatment with alkali metals. The large PAH form dianions as well.[23] The redox potential correlates with the size of the PAH.
Sources[edit]
Artificial[edit]
The dominant sources of PAHs in the environment are from human activity: wood-burning and combustion of other biofuels such as dung or crop residues contribute more than half of annual global PAH emissions, particularly due to biofuel use in India and China.[26][27] As of 2004, industrial processes and the extraction and use of fossil fuels made up slightly more than one quarter of global PAH emissions, dominating outputs in industrial countries such as the United States.[26]
A year-long sampling campaign in Athens, Greece found a third (31%) of PAH urban air pollution to be caused by wood-burning, like diesel and oil (33%) and gasoline (29%). It also found that wood-burning is responsible for nearly half (43%) of annual PAH cancer-risk (carcinogenic potential) compared to the other sources and that wintertime PAH levels were 7 times higher than in other seasons, especially if atmospheric dispersion is low.[28][29]
Lower-temperature combustion, such as tobacco smoking or wood-burning, tends to generate low molecular weight PAHs, whereas high-temperature industrial processes typically generate PAHs with higher molecular weights.[30] Incense is also a source.[31]
PAHs are typically found as complex mixtures.[32][30]
Distribution in the environment[edit]
Aquatic environments[edit]
Most PAHs are insoluble in water, which limits their mobility in the environment, although PAHs sorb to fine-grained organic-rich sediments.[44][45][46][47] Aqueous solubility of PAHs decreases approximately logarithmically as molecular mass increases.[48]
Two-ringed PAHs, and to a lesser extent three-ringed PAHs, dissolve in water, making them more available for biological uptake and degradation.[47][48][49] Further, two- to four-ringed PAHs volatilize sufficiently to appear in the atmosphere predominantly in gaseous form, although the physical state of four-ring PAHs can depend on temperature.[50][51] In contrast, compounds with five or more rings have low solubility in water and low volatility; they are therefore predominantly in solid state, bound to particulate air pollution, soils, or sediments.[47] In solid state, these compounds are less accessible for biological uptake or degradation, increasing their persistence in the environment.[48][52]
Human exposure[edit]
Human exposure varies across the globe and depends on factors such as smoking rates, fuel types in cooking, and pollution controls on power plants, industrial processes, and vehicles.[32][26][53] Developed countries with stricter air and water pollution controls, cleaner sources of cooking (i.e., gas and electricity vs. coal or biofuels), and prohibitions of public smoking tend to have lower levels of PAH exposure, while developing and undeveloped countries tend to have higher levels.[32][26][53] Surgical smoke plumes have been proven to contain PAHs in several independent research studies.[54]







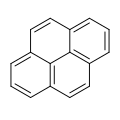

![Benzo[a]pyrene](http://upload.wikimedia.org/wikipedia/commons/thumb/f/fa/Benzo-a-pyrene.svg/120px-Benzo-a-pyrene.svg.png)

![Benzo[ghi]perylene](http://upload.wikimedia.org/wikipedia/commons/thumb/f/ff/Benzo%28ghi%29perilene.png/120px-Benzo%28ghi%29perilene.png)
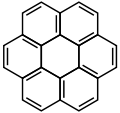

![Benzo[c]fluorene](http://upload.wikimedia.org/wikipedia/commons/thumb/4/4f/Benzocfluorene.svg/120px-Benzocfluorene.svg.png)

