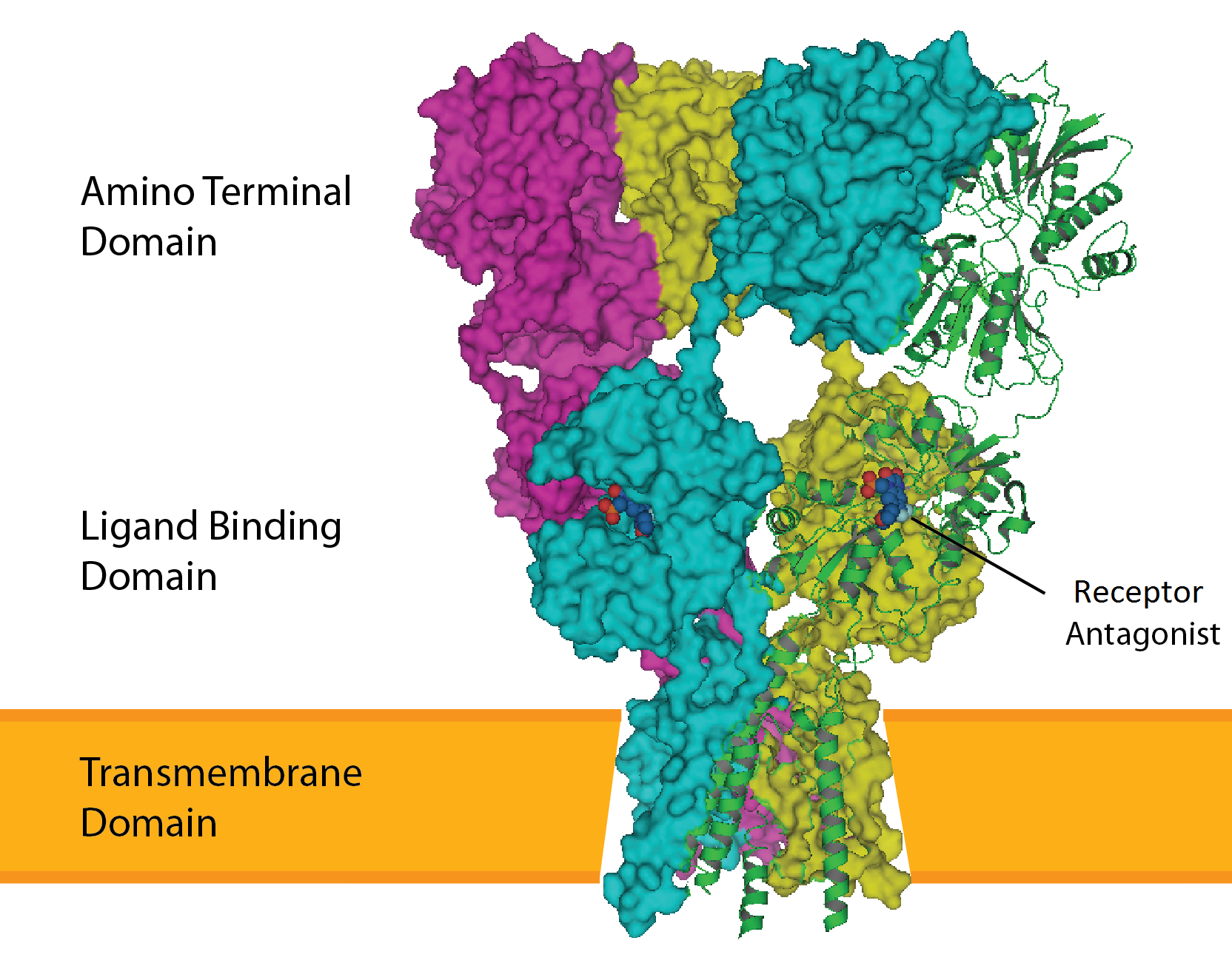
Transmembrane domain
A transmembrane domain (TMD) is a membrane-spanning protein domain. TMDs may consist of one or several alpha-helices or a transmembrane beta barrel. Because the interior of the lipid bilayer is hydrophobic, the amino acid residues in TMDs are often hydrophobic, although proteins such as membrane pumps and ion channels can contain polar residues. TMDs vary greatly in size and hydrophobicity; they may adopt organelle-specific properties.[1]
Transmembrane domains are known to perform a variety of functions. These include:
Identification of transmembrane helices[edit]
Transmembrane helices are visible in structures of membrane proteins determined by X-ray diffraction. They may also be predicted on the basis of hydrophobicity scales. Because the interior of the bilayer and the interiors of most proteins of known structure are hydrophobic, it is presumed to be a requirement of the amino acids that span a membrane that they be hydrophobic as well. However, membrane pumps and ion channels also contain numerous charged and polar residues within the generally non-polar transmembrane segments.
Using "hydrophobicity analysis" to predict transmembrane helices enables a prediction in turn of the "transmembrane topology" of a protein; i.e. prediction of what parts of it protrude into the cell, what parts protrude out, and how many times the protein chain crosses the membrane.
Transmembrane helices can also be identified in silico using the bioinformatic tool, TMHMM.[4]
