Vaccine
A vaccine is a biological preparation that provides active acquired immunity to a particular infectious or malignant disease.[1][2] The safety and effectiveness of vaccines has been widely studied and verified.[3][4] A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. The agent stimulates the body's immune system to recognize the agent as a threat, destroy it, and recognize further and destroy any of the microorganisms associated with that agent that it may encounter in the future.
For other uses, see Vaccine (disambiguation).Vaccine
Vaccines can be prophylactic (to prevent or alleviate the effects of a future infection by a natural or "wild" pathogen), or therapeutic (to fight a disease that has already occurred, such as cancer).[5][6][7][8] Some vaccines offer full sterilizing immunity, in which infection is prevented completely.[9]
The administration of vaccines is called vaccination. Vaccination is the most effective method of preventing infectious diseases;[10] widespread immunity due to vaccination is largely responsible for the worldwide eradication of smallpox and the restriction of diseases such as polio, measles, and tetanus from much of the world. The World Health Organization (WHO) reports that licensed vaccines are currently available for twenty-five different preventable infections.[11]
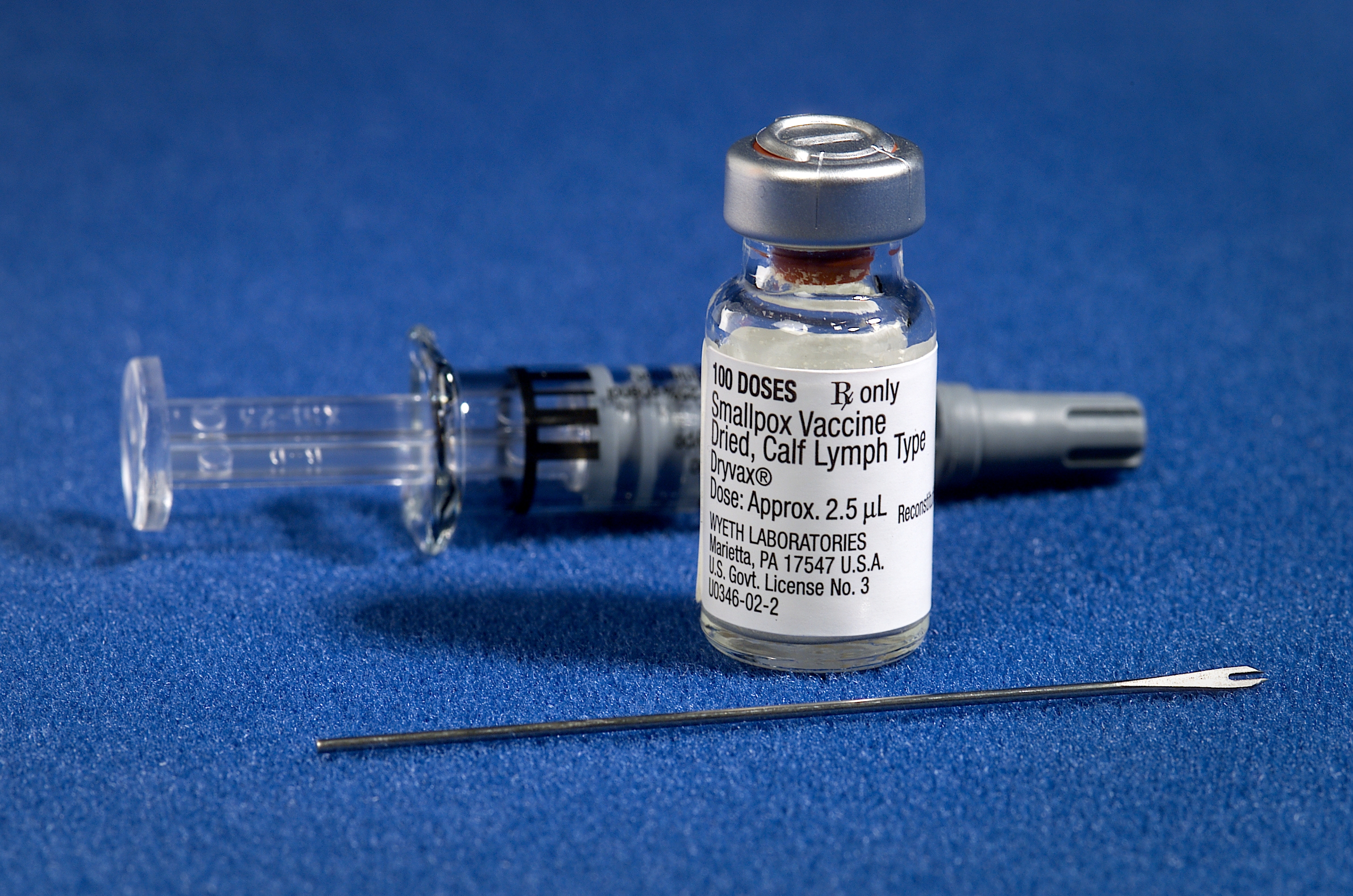
The first recorded use of inoculation to prevent smallpox occurred in the 16th century in China, with the earliest hints of the practice in China coming during the 10th century.[12] It was also the first disease for which a vaccine was produced.[13][14] The folk practice of inoculation against smallpox was brought from Turkey to Britain in 1721 by Lady Mary Wortley Montagu.[15]
The terms vaccine and vaccination are derived from Variolae vaccinae (smallpox of the cow), the term devised by Edward Jenner (who both developed the concept of vaccines and created the first vaccine) to denote cowpox. He used the phrase in 1798 for the long title of his Inquiry into the Variolae vaccinae Known as the Cow Pox, in which he described the protective effect of cowpox against smallpox.[16] In 1881, to honor Jenner, Louis Pasteur proposed that the terms should be extended to cover the new protective inoculations then being developed.[17] The science of vaccine development and production is termed vaccinology.
Nomenclature
Various fairly standardized abbreviations for vaccine names have developed, although the standardization is by no means centralized or global. For example, the vaccine names used in the United States have well-established abbreviations that are also widely known and used elsewhere. An extensive list of them provided in a sortable table and freely accessible is available at a US Centers for Disease Control and Prevention web page.[101] The page explains that "The abbreviations [in] this table (Column 3) were standardized jointly by staff of the Centers for Disease Control and Prevention, ACIP Work Groups, the editor of the Morbidity and Mortality Weekly Report (MMWR), the editor of Epidemiology and Prevention of Vaccine-Preventable Diseases (the Pink Book), ACIP members, and liaison organizations to the ACIP."[101]
Some examples are "DTaP" for diphtheria and tetanus toxoids and acellular pertussis vaccine, "DT" for diphtheria and tetanus toxoids, and "Td" for tetanus and diphtheria toxoids. At its page on tetanus vaccination,[102] the CDC further explains that "Upper-case letters in these abbreviations denote full-strength doses of diphtheria (D) and tetanus (T) toxoids and pertussis (P) vaccine. Lower-case "d" and "p" denote reduced doses of diphtheria and pertussis used in the adolescent/adult-formulations. The 'a' in DTaP and Tdap stands for 'acellular', meaning that the pertussis component contains only a part of the pertussis organism."[102]
Another list of established vaccine abbreviations is at the CDC's page called "Vaccine Acronyms and Abbreviations", with abbreviations used on U.S. immunization records.[103] The United States Adopted Name system has some conventions for the word order of vaccine names, placing head nouns first and adjectives postpositively. This is why the USAN for "OPV" is "poliovirus vaccine live oral" rather than "oral poliovirus vaccine".